

Forschungsdock CRO is a small contract research organization based in Germany. The company supports pharmaceutical and biotech companies, other CROs, and clinics in conducting clinical trials. In addition to traditional CRO services like project management, quality management, and clinical monitoring, Forschungsdock also offers seminars and training on Good Clinical Practice.
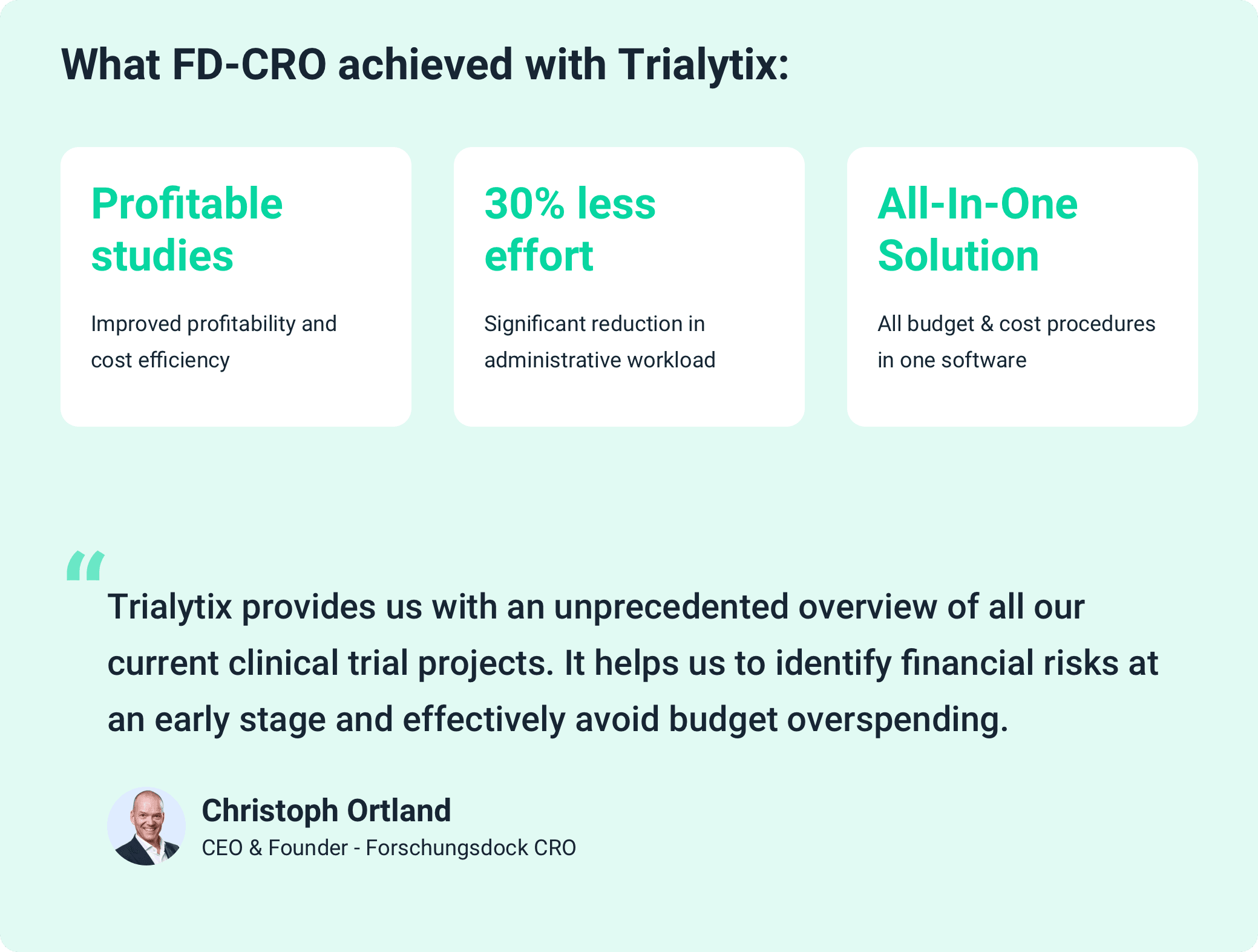
With a flexible and experienced team and a clear focus on efficiency, Forschungsdock leverages innovative solutions to manage clinical trials successfully and cost-effectively. Trialytix has established itself as an indispensable tool for effectively addressing the complex requirements of budget and cost control in clinical trials.
As Forschungsdock conducts not only clinical trials but also seminars and internal projects, the company needed a flexible tailored solution to adequately manage the different types of projects. The focus was mainly on optimizing the clinical trial projects, which require frequent adjustments to the budget, timelines and resources due to their dynamic profile.
Previously, Forschungsdock used MS Excel for operational and financial study management, which made profitability forecasting difficult and required continuous manual updates to various trackers. Another challenge was the management and analysis of study-specific incoming and outgoing invoices, which was complex and time-consuming.
To address these challenges, Forschungsdock needed a solution to reduce financial risks, increase profit margins, enhance transparency, and sustainably improve study management efficiency.

By implementing Trialytix, Forschungsdock introduced a customized all-in-one software solution designed specifically to meet the needs of modern CROs. Trialytix provides comprehensive features for the operational management and budget control of clinical trial projects. The software allows for precise management of internal and external cost sources, along with the real-time monitoring of study profitability. This enables the early detection of budget risks and proactive mitigation measures.
Trialytix also simplifies the management of all study-related financial transactions, such as payments to external monitors or ethics committee fees. This centralized approach ensures complete oversight and fosters transparency. Invoices and payments can be processed seamlessly as milestones, partial payments, or pass-through costs and are fully integrated within Trialytix. The platform also offers a detailed cash flow forecast, providing a solid foundation for financial decision-making at both the study and organizational levels.
The intuitive user interface of Trialytix presents financial data through clear dashboards and detailed reports, ensuring a high degree of transparency. This enables Forschungsdock's team to make data-driven decisions. Financial and operational risks can be identified and minimized through continuous cost monitoring. Meanwhile, the software's flexibility allows for quick adjustments to budgets and resources to meet changing requirements without compromising project profitability.

The adoption of Trialytix has fundamentally digitized and optimized the financial procedures of Forschungsdock's clinical trials. The platform delivers an innovative solution that enhances transparency, boosts profitability, and significantly increases efficiency. Thanks to automated workflows and flexible customization options, Forschungsdock has improved productivity while focusing more on its core activities. Implementing Trialytix was a strategic step to sustainably meet the increasing demands of clinical trial projects in the long term.